Arc Discharge of MWF
PRODUCT FOCUS![[Dividing Line Image]](/mwf/pictures/div.gif)
POWERPULSE® TREATMENT OF METALWORKING FLUID
by Jerome R. Fishback
Chief R&D Engineer
Scientific Utilization, Inc.
SEPTEMBER 2001:
Introduction
Metalworking fluids are an essential product in numerous industries, particularly in auto and truck manufacturing, parts plants, aerospace and rail industries, foundries, metal manufacturing, and marine and auto servicing. The primary function of these metalworking fluids is cooling and lubrication to decrease tool wear and to achieve the desired finish of the workpiece. A secondary function is to flush away chips and metal fines from the tool/workpiece interface. Metalworking fluids are a complex mixture of water, petroleum products, vegetable/animal fats, and various additives. An important characteristic of the metalworking fluid is a stable emulsion or the use of water-soluble oils so that there is a homogeneous product that provides both cooling and lubrication.
Because of the organic component of these fluids, microorganisms flourish. These microorganisms cause deterioration of the metalworking fluid, and produce strong odors and toxic by-products. Ultimately, a complete change of the fluid is required. This involves downtime of machinery, draining the fluid, adding a new batch of fluid, and disposal of the old fluid, all at considerable cost to the company. At this time, chemical biocides are commonly used to control microbial growth in metalworking fluids. Machine tool workers are therefore at risk from exposure to fluids and aerosol mists that can contain toxic bacterial by-products, as well as the disinfectant biocides used to combat the microbes.
An alternative, non-chemical disinfection process would reduce worker’s health risks, and could ultimately reduce the frequency of fluid changes, with significant cost savings for companies. The SUI PulsePower® process has this potential. In bench scale tests, the PulsePower® process has proven to significantly decrease microorganisms in metalworking fluid. This report describes the procedures and results from three tests conducted in June 2001.
Metalworking Fluid
June 2001 tests
Test 1 – June, 2001: PPS-4000; four cross-flow, arc discharge chambers; recirculated fluid (June 5-11, 2001)
Procedure: A batch of used metalworking fluid was treated with the PPS-4000 system connected to four arc discharge chambers over a period of 3 days. The chambers consisted of wire feed electrodes and a cross-flow configuration. This test was a series of eight treatments per day, each treatment being 30 minutes of PulsePower® arc discharge exposure and 30 minutes without arc discharge. The same batch of metalworking fluid was used for the duration of the test, and the fluid was recirculated continuously during the testing period. The fluid was covered but uncirculated for the 16 hour period between daily treatments. A control sample was taken (untreated) the first day, and prior to treatment on the second day. Each day, samples were collected at the end of each arc discharge treatment (samples taken every hour).
Laboratory techniques: The samples were plated for microbial growth, using the "Heterotrophic plate count" (previously called "Standard plate count"). The samples were diluted with sterile buffered water and plated to obtain standard data in the range of 20 to 500 colonies per plate. This range is considered to be countable and an accurate representation of the bacterial levels being measured. The dilutions and plating aliquots are always based on an estimate of what levels the analyst expects in the samples. Sometimes the dilutions miss the countable range, as in day 2 results, below. The standard growth for Heterotrophic plate counts is 48 hours. However, our previous work with the microbes in metalworking fluid showed that after 48 hours many colonies are very small. To better see these colonies, the plates were allowed 4 days growth before counting.
The results are given in "Colony Forming Units per milliliter" (CFU/ml). Each colony is the growth resulting from one microorganism. Therefore, each colony on the plate counts as one microorganism in the original sample. For example, if there are 20 colonies on a plate, the sample applied to that plate contained 20 microorganisms.
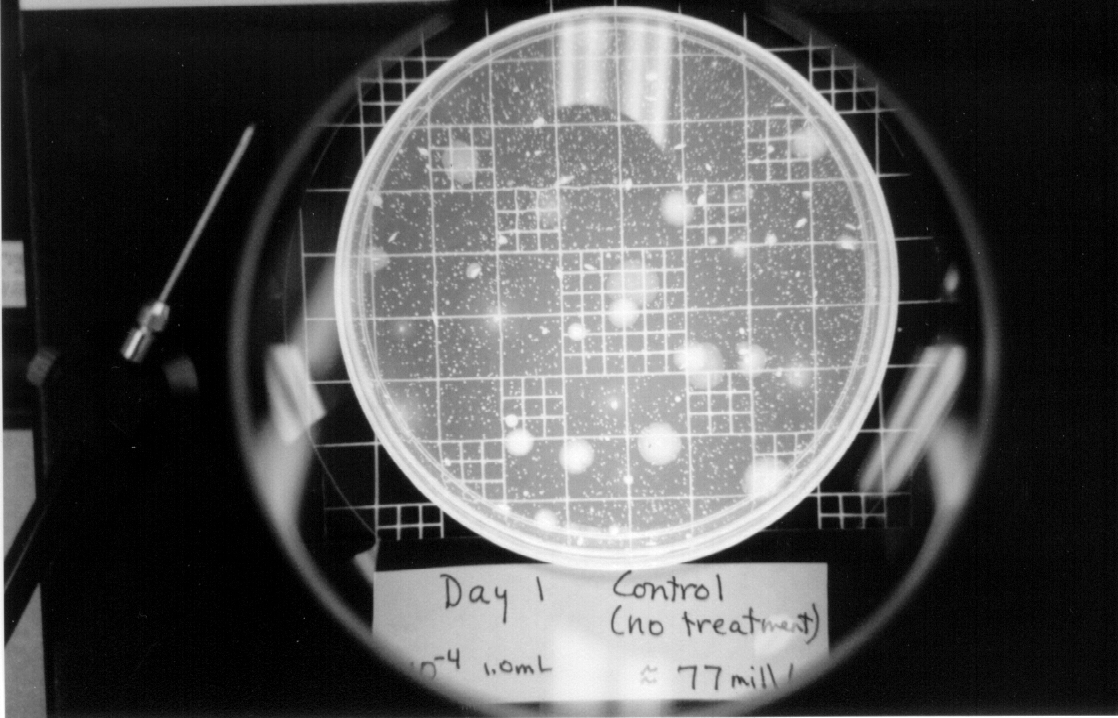
Pictures of three plates are shown in the Appendix, one for the test control, one at the end of the first day of testing, and one at the end of the entire test.
Results: Heterotrophic Plate Count
Day 1
Sample dilutions and aliquots: 10-4 (1.0, 0.1 mL) and 10-6 (1.0, 0.1 mL).
For this day of testing, countable plates were obtained on all but the last three treatments. For these samples, an estimated CFU/ml is shown. The data are presented below in Table 1.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Control |
10^-6 |
1.0 |
77 |
7.7 x 10^7 |
|
Treated 1 |
10^-4 |
0.1 |
330 |
3.3 x 10^7 |
|
Treated 2 |
10^-4 |
0.1 |
273 |
2.7 x 10^7 |
|
Treated 3 |
10^-4 |
0.1 |
133 |
1.3 x 10^7 |
|
Treated 4 |
10^-4 |
0.1 |
36 |
3.6 x 10^6 |
|
Treated 5 |
10^-4 |
1.0 |
28 |
2.8 x 10^5 |
|
Treated 6-8 |
10^-4 |
1.0 |
all 0 or 1 |
< 1 x 10^4 est |
Table 1 – Plate count data for first day
Day 2
Sample dilutions and aliquots: 10-4 (1.0, 0.1 mL) and 10-6 (1.0, 0.1 mL)
For this day of testing, all plates had 0 to 1 colony per plate. Based on 0 or 1 colony on 10-4, 1.0 ml plates, the fluid had a heterotrophic plate count of <1 x 104 colonies per mL.
Day 3
Sample dilutions and aliquots: Straight (0.1), 10-2 (1.0, 0.1 mL) and 10-4 (1.0, 0.1 mL).
For this day of testing, all plates were outside of the standard countable range. Due to the opacity of the metal working fluid, a larger aliquot was not feasible due to the resulting cloudiness of the plate media. For reference, the data are presented below in Table 2.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Treated 1 |
Straight |
0.1 |
2 |
20 ± 50% |
|
Treated 2 |
Straight |
0.1 |
7 |
70 ± 50% |
|
Treated 3 |
Straight |
0.1 |
4 |
40 ± 50% |
|
Treated 4 |
Straight |
0.1 |
0 |
£ 5 |
|
Treated 5 |
Straight |
0.1 |
3 |
30 ± 50% |
|
Treated 6 |
Straight |
0.1 |
2 |
20 ± 50% |
|
Treated 7 |
Straight |
0.1 |
0 |
£ 5 |
Table 2 – Plate count data for third day
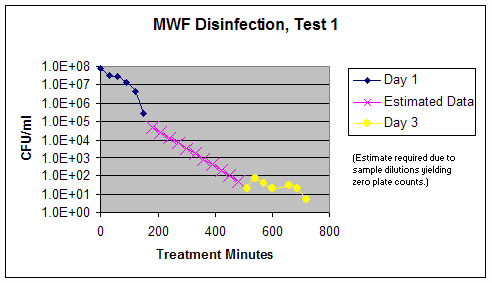
Graph 1 – Test 1 Composite Disinfection Results
Conclusions: During this test, the microbial counts decreased significantly in the metalworking fluid, from levels of 107 colonies per mL in the untreated fluid to levels of less than or equal to 101 colonies per mL in the treated fluid. This is considered to be a 6-log kill of these microorganisms. Because the kill rate was unknown at the beginning of the test, sample dilutions and aliquots did not always produce microbiological plates in a "countable" range. This is represented by the "estimated data" shown in Graph 1.
As a follow up investigation to Test 1, after eight days, samples were plated from the Test 1 reservoir of treated fluid, and from the last test sample taken during that test series. It was observed that the sample from the reservoir showed significant regrowth (~2.2E8 CFU/ml), attributable to the fact that some viable bacteria could be expected to remain somewhere in the system (e.g. on walls and/or crevices). Conversely, the final sample, which was collected in a sterile container, showed zero regrowth with no colonies visible on any of the plates.
The entire test was scheduled to be repeated in Test 2, during the week of June 12-18, 2001; and Test 3, with slight modifications, during the days of June 21-22, 2001. Dilutions and aliquots were adjusted accordingly for Test 2 and Test 3.
Test 2 – June, 2001: PPS-4000; four cross-flow, arc discharge chambers; recirculated fluid (June 13-15, 2001)
Procedure: The test from 5-11 June 2001 (Test 1, above) was repeated in its entirety during 13-15 June 2001, using a new batch of used metalworking fluid. In test 2, as in the previous test, a batch of metalworking fluid was treated with the PPS-4000 system connected to four wire-feed, cross-flow arc discharge chambers over a period of 3 days. This test consisted of a series of eight treatments per day, each treatment being 30 minutes of PulsePower® arc discharge exposure and 30 minutes without arc discharge. The same batch of metalworking fluid was used for the duration of the test, and the fluid was recirculated continuously during the testing period. The fluid was covered but uncirculated for the 16 hour period between daily treatments. A control sample was taken (untreated) the first day, and prior to treatment on the second and third day. Each day, samples were collected at the end of each arc discharge treatment (samples taken every hour).
Laboratory techniques: The samples were plated for microbial growth, using the "Heterotrophic plate count" (previously called "Standard plate count"). The samples were diluted with sterile buffered water and plated to obtain standard data in the range of 20 to 500 colonies per plate. This range is considered to be countable and an accurate representation of the bacterial levels being measured. The dilutions and plating aliquots were estimated from the results of test 1. The plates were allowed 4 days growth before counting.
Results: Heterotrophic Plate Count
Day 1
Sample dilutions and aliquots: 10-2 (1.0, 0.1 mL), 10-4 (1.0, 0.1 mL) and
10-6 (1.0, 0.1 mL).
For this day of testing, countable plates were obtained for all samples. The data are presented below in Table 3 and in Graph 2.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Control |
10^-6 |
1.0 |
59 |
5.9 x 10^7 |
|
Treated 1 |
10^-6 |
1.0 |
34 |
3.4 x 10^7 |
|
Treated 2 |
10^-4 |
0.1 |
278 |
2.8 x 10^7 |
|
Treated 3 |
10^-4 |
0.1 |
119 |
1.2 x 10^7 |
|
Treated 4 |
10^-4 |
0.1 |
43 |
4.3 x 10^6 |
|
Treated 5 |
10^-4 |
1.0 |
156 |
1.6 x 10^6 |
|
Treated 6 |
10^-4 |
1.0 |
45 |
4.5 x 10^5 |
|
Treated 7 |
10^-2 |
0.1 |
51 |
5.1 x 10^4 |
|
Treated 8 |
10^-2 |
1.0 |
68 |
6.8 x 10^3 |
Table 3 – Plate count data for first day
Day 2
Sample dilutions and aliquots: Straight (0.1 mL), 10-2 (1.0, 0.1 mL) and
10-4 (1.0).
For this day of testing, countable plates were obtained for all samples. The data are presented below in Table 4.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Control |
10^-2 |
1.0 |
74 |
7.4 x 10^3 |
|
Treated 1 |
Straight |
0.1 |
280 |
2.8 x 10^3 |
|
Treated 2 |
Straight |
0.1 |
83 |
8.3 x 10^2 |
|
Treated 3 |
Straight |
0.1 |
51 |
5.1 x 10^2 |
|
Treated 4 |
Straight |
0.1 |
11 |
1.1 x 10^2 |
|
Treated 5 |
Straight |
0.1 |
32 |
3.2 x 10^2 |
|
Treated 6 |
Straight |
0.1 |
28 |
2.8 x 10^2 |
|
Treated 7 |
Straight |
0.1 |
37 |
3.7 x 10^2 |
|
Treated 8 |
Straight |
0.1 |
38 |
3.8 x 10^2 |
Table 4 – Plate count data for second day
Day 3
Sample dilutions and aliquots: Straight (0.1), and 10-2 (1.0, 0.1 mL).
For this day of testing, countable plates were obtained for all samples. The data are presented below in Table 5.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Control |
Straight |
0.1 |
41 |
4.1 x 10^2 |
|
Treated 1 |
Straight |
0.1 |
140 |
1.4 x 10^3 |
|
Treated 2 |
Straight |
0.1 |
71 |
7.1 x 10^2 |
|
Treated 3 |
Straight |
0.1 |
56 |
5.6 x 10^2 |
|
Treated 4 |
Straight |
0.1 |
47 |
4.7 x 10^2 |
|
Treated 5 |
Straight |
0.1 |
40 |
4.0 x 10^2 |
|
Treated 6 |
Straight |
0.1 |
75 |
7.5 x 10^2 |
|
Treated 7 |
Straight |
0.1 |
40 |
4.0 x 10^2 |
|
Treated 8 |
Straight |
0.1 |
22 |
2.2 x 10^2 |
Table 5 – Plate count data for third day
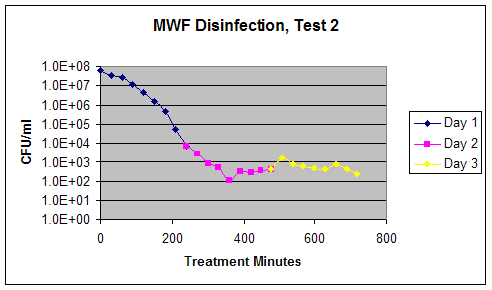
Graph 2 – Test 2 Composite Disinfection Results
Conclusions: During this test, the microbial counts decreased significantly in the metalworking fluid, from levels of 107 colonies per mL in the untreated fluid to levels of less than or equal to 102 colonies per mL in the treated fluid. This is a 5-log kill of these microorganisms. It can be noted that the bacterial levels seemed to rebound and stabilize at a higher level than that observed during Test 1 (~500 CFU/ml for Test 2 versus ~50 CFU/ml for Test 1).
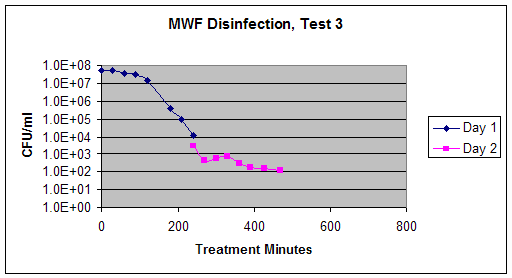
Test 3 – June, 2001: PPS-4000; four cross-flow, arc discharge chambers; recirculated fluid (June 21-22, 2001)
Procedure: The test from 5-11 June 2001 (Test 1, above) was repeated again but with slight modifications during 21-22 June 2001. In test 3, a new batch of used metalworking fluid was treated with the PPS-4000 system connected to four wire-feed, cross-flow arc discharge chambers over a period of 2 days instead of 3 days. This test consisted of a series of seven treatments per day, each treatment being 30 minutes of PulsePower® arc discharge exposure and 30 minutes without arc discharge. One exception to this procedure is that the 5th treatment of Day 1 was 60 minutes. That treatment period was followed by a corresponding non-treatment period of 60 minutes. The same batch of metalworking fluid was used for the duration of the test, and the fluid was recirculated continuously during the testing period. The fluid was covered but uncirculated for the 16 hour period between the two days of treatment. A control sample was taken (untreated) the first day, and prior to treatment on the second day. Each day, samples were collected at the end of each arc discharge treatment (samples taken every hour).
Laboratory techniques: The samples were plated for microbial growth, using the "Heterotrophic plate count" (previously called "Standard plate count"). The samples were diluted with sterile buffered water and plated to obtain standard data in the range of 20 to 500 colonies per plate. This range is considered to be countable and an accurate representation of the bacterial levels being measured. The dilutions and plating aliquots were estimated from the results of Test 1 and Test 2. The plates were allowed 4 days growth before counting.
Results: Heterotrophic Plate Count
Day 1
Sample dilutions and aliquots: 10-2 (1.0, 0.1 mL), 10-4 (1.0, 0.1 mL) and
10-6 (1.0, 0.1 mL).
For this day of testing, countable plates were obtained for all samples. The data are presented below in Table 6 and in Graph 3.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Control |
10^-6 |
1.0 |
51 |
5.1 x 10^7 |
|
Treated 1 |
10^-6 |
1.0 |
51 |
5.1 x 10^7 |
|
Treated 2 |
10^-6 |
1.0 |
36 |
3.6 x 10^7 |
|
Treated 3 |
10^-4 |
0.1 |
310 |
3.1 x 10^7 |
|
Treated 4 |
10^-4 |
0.1 |
147 |
1.5 x 10^7 |
|
Treated 5 |
10^-2 |
0.1 |
323 |
3.2 x 10^5 |
|
Treated 6 |
10^-2 |
0.1 |
102 |
1.0 x 10^5 |
|
Treated 7 |
10^-2 |
1.0 |
119 |
1.2 x 10^4 |
Table 6 – Plate count data for first day
Day 2
Sample dilutions and aliquots: Straight (0.1 mL), and 10-2 (1.0, 0.1 mL).
For this day of testing, countable plates were obtained for all samples. The data are presented below in Table 7.
|
Sample |
Dilution |
Aliquot |
Plate Count |
CFU/ml |
|
Control |
10^-2 |
1.0 |
28 |
2.8 x 10^3 |
|
Treated 1 |
Straight |
0.1 |
44 |
4.4 x 10^2 |
|
Treated 2 |
Straight |
0.1 |
55 |
5.5 x 10^2 |
|
Treated 3 |
Straight |
0.1 |
74 |
7.4 x 10^2 |
|
Treated 4 |
Straight |
0.1 |
29 |
2.9 x 10^2 |
|
Treated 5 |
Straight |
0.1 |
18 |
1.8 x 10^2 |
|
Treated 6 |
Straight |
0.1 |
16 |
1.6 x 10^2 |
|
Treated 7 |
Straight |
0.1 |
12 |
1.2 x 10^2 |
Table 7 – Plate count data for second day
Graph 3 – Test 3 Composite Disinfection Results
Conclusions: During this test, the microbial counts decreased significantly in the metalworking fluid, from levels of 107 colonies per mL in the untreated fluid to levels of 102 colonies per mL in the treated fluid. This is a 5-log kill of these microorganisms.
Summary and Conclusions
Three bench-scale tests of the PulsePower® treatment of metalworking fluids were performed during June 2001. In all three tests, the level of microorganisms ("Heterotrophic Plate Count") was decreased significantly after PulsePower® treatment. Initial bacterial levels before treatment were 107 CFU/mL, and final levels after treatment were 101 to 102 CFU/mL. A number of observations can be made from the data.
First, as can be seen in the graphs of the data, a linear log kill rate was not observed. The kill rate starts off slowly during the first few treatments of Day 1. During the latter half of Day 1, the kill rate increases by a factor of 2 to 3. During Days 2 and 3, the kill rate levels off, until continued treatment does not appear to reduce the bacterial levels.
Second, there is little regrowth of microorganims overnight during the 16 hour period between treatment days. This is observed for all three tests, as can be seen by comparing the plate count data for the last treated sample of one day with the control data of the next day.
These bench scale tests successfully demonstrated the biocidal properties of PulsePower® treatment when applied to metalworking fluids. It may be noted that, in actual installations, the desired bacterial level is often in the range of 105 CFU/ml, three orders of magnitude higher than the levels achieved in these tests.
Appendix